Description
This detection kit is used for clinical in vitro quantitative detection of cardiac troponin I (cTnI) content in human serum, plasma or whole blood.
Cardiac troponin is composed of three subunits of cardiac troponin I, T, and C, and is a regulatory protein of cardiac muscle contraction. When ischemia or other reasons (such as myocarditis) cause myocardial damage, cTnl will increase in the blood. Cardiac troponin I has a high degree of myocardial specificity and sensitivity, thus becoming the “gold standard” for the diagnosis of acute myocardial infarction (AMI). Clinical studies have confirmed that all patients with suspected acute coronary syndrome (ACS) should be tested for troponin to assist in the diagnosis of the disease. Therefore, cTnl can be used for the early diagnosis and risk stratification of acute coronary syndrome (ACS), especially acute myocardial infarction (AMI); it can be used to evaluate the area of myocardial ischemia damage and the evaluation of clinical treatment effects; it can be used for infection, radiotherapy/chemotherapy Auxiliary diagnosis and treatment monitoring of injury diseases caused by various reasons such as poisoning, etc. At present, clinical and laboratory methods for detecting cardiac troponin I include colloidal gold method, immunofluorescence method, chemiluminescence method, latex enhanced immunoturbidimetric method and so on.
Detection principle
Kit principle: Three strains of cTnl monoclonal antibodies with high specificity and sensitivity, among which monoclonal antibody I is a fluorescently labeled antibody, which is pre-coated on a fluorescent pad, and monoclonal antibody II and monoclonal antibody III are capture antibodies. In the detection area, the quality control area is coated with rabbit anti-mouse IgG antibody, and the antigen-antibody reaction and fluorescence immunochromatography technology are used to quantitatively detect the content of cTnl in human blood.
The working principle of the supporting instrument: The measuring system of the instrument automatically performs the detection of the binding area of the label and the analyte on the test card after the reaction.
Scan to obtain optical signal. Then the optical signal is measured and analyzed, and the concentration of the measured substance is obtained quantitatively.
Specification:25T
Kit composition
Cardiac Troponin I Test Card
manual
straw
SD card
Buffer
Validity period and storage conditions
The test card is stored in a sealed state at 4~30℃, and the validity period is 18 months.
Sample requirements
- For human serum, plasma or whole blood samples, other body fluids and samples may not get accurate results.
- The venous blood should be collected under aseptic conditions. It is recommended to use human serum or plasma for testing.
- Blood and whole blood samples can use heparin, EDTA or sodium citrate anticoagulant.
- After clinical blood samples are collected, the test must be completed within 4 hours at room temperature; serum plasma can be stored at 2-8℃ for 5 days; stored below -20℃ for 6 months. Whole blood samples should not be frozen, stored at 2~8℃, and can be stored for 3 days. Avoid heating and inactivating samples, and hemolyzed samples should be discarded.
- The sample must be returned to room temperature before testing. The frozen samples must be completely thawed, rewarmed, and evenly mixed before use. Avoid repeated freezing and thawing. It is recommended that the sample be frozen and thawed no more than once.
Testing method
Before using this reagent, you must read the instructions of the reagents carefully, and perform the shut-off operation strictly in accordance with the instructions of the reagents, otherwise reliable results cannot be guaranteed.
- Preparation
Return the test card, sample, and buffer to room temperature (15~30 °C) before testing. It is recommended to unpack the test card after returning to room temperature
, And use it as soon as possible within the validity period to prevent the test card from getting wet.
- Calibration
Confirm that the batch number of the SD card matches the test card, perform SD card calibration, and calibrate the instrument parameters (see the instrument manual for details).
- Sample addition method and detection
①According to the type of sample, select the sample mode on the analyzer: “serum/plasma” mode or “whole blood” mode.
- Serum and plasma: draw 100ul of serum or plasma sample, drop it vertically to the sample point of the test card, and start timing.
- Whole blood: draw 100 ul whole blood sample, drop it vertically to the sample point of the test card, immediately add a drop of whole blood buffer (20ul-30ul) to the sample point, and start timing.
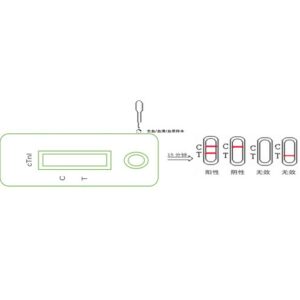
②After 10 minutes, insert the test card into the carrier of the fluorescence immunoassay analyzer, press the “measure” button, the instrument will automatically detect and print the results.
Positive judgment value or reference interval
The statistical analysis of the determination results of 1000 healthy human serum samples showed that the upper limit of the cTnl reference interval took the 99th percentile as 0.10ng/ml. The reference interval for plasma and whole blood samples is the same as the reference interval for serum samples.
Each laboratory shall determine the applicability of the reference interval through experiments, and establish the reference interval of the laboratory when necessary.
Interpretation of test results
Use a fluorescent immunoquantitative analyzer to analyze the test card and issue a quantitative test result.
When the sample concentration exceeds the upper limit of detection, calf serum or negative samples should be used to dilute high-concentration samples, and the maximum dilution factor is 5 times.
The hemoglobin, triglycerides and bilirubin in the sample will interfere with the test results, and the maximum allowable concentrations are 5 g/L, 10 g/L and 0.2 g/L.
Kit performance
- Lowest detection limit
The minimum detection limit should not be higher than 0.1ng/ml.
- Linear range
In the interval of 0.1-50ng/ml, the linear correlation coefficient r≥0.990.
- Accuracy
The relative deviation should not be greater than 20%.
- Repeatability
The repeatability should not be greater than 10%.
Limitations of the test method
- This kit is limited to in vitro diagnostic use.
- The test results of this kit are for clinical reference only, and cannot be used alone as a basis for confirming or excluding cases. In order to achieve diagnostic purposes, the test results should be combined with clinical examination, medical history and other examination results.
Precautions
- The straws cannot be mixed to avoid cross-contamination.
- After the test card is unpacked, the test should be carried out as soon as possible to avoid leaving it in the air for too long, which may cause moisture.
- The test card can be sealed and stored at room temperature. Beware of moisture. The test card stored at low temperature should be equilibrated to room temperature before use.
- This product contains sodium azide as a preservative. Sodium azide can react with the copper pipe or lead pipe of the drainage pipe and cause an explosion.
Please handle properly in accordance with the relevant local regulations.
- For those substances that contain the source of infection or suspected of containing the source of infection, there should be appropriate biosafety assurance procedures. The following are relevant precautions:
☆Wear gloves to handle samples or disinfect reagents.
☆ Use disinfectant to disinfect the spilled samples or reagents.
☆Disinfect and dispose of all specimens, reagents and potential contaminants in accordance with relevant local regulations.
- The calibrator of cardiac troponin I is traceable to the international standard material SRM2921.
- The company has the final right to interpret matters related to the uncertainty of the calibrator.